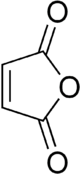Maleic anhydride
| Maleic anhydride[1] | |
|---|---|
| IUPAC name | Furan-2,5-dione |
| Identifiers | |
| InChI | InChI=1/C4H2O3/c5-3-1-2-4(6)7-3/h1-2H |
| InChIKey | FPYJFEHAWHCUMM-UHFFFAOYAP |
| Standard InChI | InChI=1S/C4H2O3/c5-3-1-2-4(6)7-3/h1-2H |
| Standard InChIKey | FPYJFEHAWHCUMM-UHFFFAOYSA-N |
| CAS number | [] |
| EC number | |
| RTECS | UE5950000 |
| ChemSpider | |
| SMILES | |
| Properties[2][3][4][5] | |
| Chemical formula | C4H2O3 |
| Molar mass | 98.057 g/mol |
| Appearance | White crystals |
| Density | 1.48 g/cm3 |
| Melting point |
53 °C, 326.0 K, 127 °F |
| Boiling point |
200 °C, 473.2 K, 392 °F |
| Triple point | 52.57 ºC (325.72 K) |
| Solubility in water | reacts |
| Thermochemistry[6] | |
| Std enthalpy of formation ΔfH |
470.0 kJ/mol |
| Std enthalpy of combustion ΔcH |
1389.9 kJ/mol |
| Std enthalpy of fusion ΔfusH |
13.6 kJ/mol |
| Std enthalpy of vaporization ΔvapH |
54.8 kJ/mol |
| Hazards[2][4][7] | |
| Material safety data sheet (MSDS) | ICSC |
| EU index number | 607-096-00-9 |
| GHS pictograms |   
|
| GHS signal word | DANGER |
| GHS hazard statements | H302, H314, H334, H317 |
| Flash point | 102 °C (216 ºF) |
| Autoignition temp. | 477 ºC (891 ºF) |
| Explosive limits | 1.4–7.1% |
| PEL (U.S.) | 1 mg/m3 TWA |
| IDLH level | 10 mg/m3 TWA |
| Related compounds | |
| Other acid anhydrides | Succinic anhydride |
| Other compounds | Maleic acid |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Maleic anhydride (cis-butenedioic anhydride, toxilic anhydride, dihydro-2,5-dioxofuran) is an organic compound with the formula C4H2O3. In its pure state it is a colourless or white solid with an acrid odour.
Maleic anhydride was traditionally manufactured by the oxidation of benzene or other aromatic compounds. As of 2006, only a few smaller plants continue to use benzene; due to rising benzene prices, most maleic anhydride plants now use n-butane as a feedstock:
- 2 CH3CH2CH2CH3 + 7 O2 → 2 C2H2(CO)2O + 8 H2O
Characteristic reactions
The chemistry of maleic anhydride is very rich, reflecting its ready availability and bifunctional reactivity.
- It hydrolyzes, producing maleic acid, cis-HO2CCH=CHCO2H. With alcohols, the half-ester is generated, e.g., cis-HO2CCH=CHCO2CH3.
- Maleic anhydride is a potent dienophile in Diels-Alder reactions.
- Maleic anhydride (MA) is an excellent ligand for low-valent metal complexes, examples being Pt(PPh3)2(MA) and Fe(CO)4(MA).
References
- ↑ The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 11th ed.; Merck, 1989. ISBN 091191028X, 5586
- ↑ 2.0 2.1 Maleic anhydride; International Chemical Safety Card 0799; International Labour Organization: Geneva, October 2005, <http://www.inchem.org/documents/icsc/icsc/eics0799.htm>.
- ↑ 2,5-Furandione. In NIST Chemistry WebBook; National Institute for Standards and Technology, <http://webbook.nist.gov/cgi/inchi/InChI%3D1S/C4H2O3/c5-3-1-2-4(6)7-3/h1-2H>. (accessed 21 August 2009).
- ↑ 4.0 4.1 Maleic anhydride. In Pocket Guide to Chemical Hazards; U.S. Department of Health and Human Services (NIOSH) Publication No. 2005-149; Government Printing Office: Washington, DC, 2005. ISBN 9780160727511, <http://www.cdc.gov/niosh/npg/npgd0376.html>.
- ↑ DeWit, H. G. M.; DeKruif, C. G.; Van Miltenburg, J. C. Thermodynamic properties of molecular organic crystals containing organic crystals containing nitrogen, oxygen, and sulfur. II. Molar heat capacities of eight compounds by adiabatic calorimetry. J. Chem. Thermodynam. 1983, 15 (9), 891–902. DOI: 10.1016/0021-9614(83)90095-2.
- ↑ Winstrom, Leon O.; Kulp, Laurence Vapor pressure of maleic anhydride. Ind. Eng. Chem. 1949, 41 (11), 1584-86. DOI: 10.1021/ie50479a044. Parks, George S.; Mosley, John R.; Peterson, Peter V., Jr. Heats of combustion and formation of some organic compounds containing oxygen. J. Chem. Phys. 1950, 18 (1), 152–53. DOI: 10.1063/1.1747444. Mastrangelo, S. V. R. Adiabatic calorimeter for determination of cryoscopic data. Anal. Chem. 1957, 29 (5), 841–45. DOI: 10.1021/ac60125a032. Wilhoit, R. C.; Shiao, Daniel Thermochemistry of biologically important compounds. Heats of combustion of solid organic acids. J. Chem. Eng. Data 1964, 9 (4), 595–99. DOI: 10.1021/je60023a038. DeWit, H. G. M.; Offringa, J. C. A.; De Kruif, C. G.; Van Miltenburg, J. C. Thermodynamic properties of molecular organic crystals containing nitrogen, oxygen and sulfur. III. Molar heat capacities measured by differential scanning calorimetry. Thermochim. Acta 1983, 65 (1), 43–51. DOI: 10.1016/0040-6031(83)80006-9.
- ↑ Index no. 607-096-00-9 of Annex VI, Part 3, to Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. OJEU L353, 31.12.2008, pp 1–1355 at p 542.
External links
- International Chemical Safety Card 0799
- NIOSH Pocket Guide to Chemical Hazards 0376
- Chronic toxicity summary
- Maleic anhydride at Occupational Safety & Health Administration
| Error creating thumbnail: Unable to save thumbnail to destination | |
This page was originally imported from Wikipedia, specifically this version of the article "Maleic anhydride". Please see the history page on Wikipedia for the original authors. This WikiChem article may have been modified since it was imported. It is licensed under the Creative Commons Attribution–Share Alike 3.0 Unported license. |