Aluminium phosphide
| Aluminium phosphide | |
|---|---|
| |
| Other names | Aluminium(III) phosphide Aluminium monophosphide Phostoxin Fumitoxin |
| Identifiers | |
| InChI | InChI=1/Al.P/rAlP/c1-2 |
| InChIKey | PPNXXZIBFHTHDM-LQQCNYPFAR |
| Standard InChI | InChI=1S/Al.P |
| Standard InChIKey | PPNXXZIBFHTHDM-UHFFFAOYSA-N |
| CAS number | [] |
| EC number | |
| RTECS | BD1400000 |
| ChemSpider | |
| PubChem | |
| Properties[1] | |
| Chemical formula | AlP |
| Molar mass | 57.955 g/mol |
| Appearance | yellow or gray crystals |
| Density | 2.85 g/cm3, solid |
| Melting point |
2530 °C |
| Solubility in water | reacts to release phosphine |
| Band gap | 2.5 eV (indirect) |
| Refractive index (nD) | 2.75 (IR), ~3 (visible) |
| Structure | |
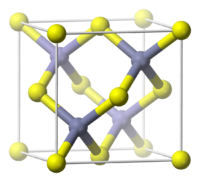
| Crystal structure | Sphalerite (cubic) |
| Lattice constant | a = 545.1 pm |
| Coordination geometry | Tetrahedral (Al3+) Tetrahedral (P3−) |
| Hazards[2] | |
| Material safety data sheet (MSDS) | ICSC |
| EU index number | 015-004-00-8 |
| GHS pictograms |   
|
| GHS signal word | DANGER |
| GHS hazard statements | H260, H300, H400 |
| Flash point | >800 °C |
| LD50 | 11.5 mg/kg |
| Related compounds | |
| Other anions | Aluminium nitride Aluminium arsenide Aluminium antimonide |
| Other cations | Gallium phosphide Indium phosphide Thallium phosphide |
| Other compounds | Aluminium gallium phosphide Aluminium gallium indium phosphide |
| Template:Tick(what is this?) (verify) Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Aluminium phosphide is the chemical compound with the empirical formula AlP. This colourless solid is generally sold as a grey-green-yellow powder due to the presence of impurities arising from hydrolysis and oxidation. This material is a wide band gap semiconductor and is used as a fumigant.
Contents
Structure, synthesis, and chemical properties
AlP crystallizes in the cubic zinc blende lattice, wherein all atoms have tetrahedral coordination. Related materials crystallize similarly, including GaAs. At pressures of 14-17 GPa, AlP transforms into a rocksalt phase.[1]
Crude aluminium phosphide can be prepared in the laboratory by igniting a mixture of red phosphorus and powdered aluminium.[3]
Aluminium phosphide reacts with water or acids to release phosphine.[4]
- AlP + 3 H2O → Al(OH)3 + PH3
- AlP + 3 H+ → Al3+ + PH3
Physical properties
Aluminium phosphide has a hardness of 5.5 on the Mohs scale.[1]
Pesticide
AlP is used as a rodenticide, insecticide, and fumigant for stored cereal grains. It is used to kill small verminous mammals such as moles, rabbits, and rodents. The tablets or pellets typically also contain other chemicals that evolve ammonia which helps to reduce the potential for spontaneous ignition or explosion of the phosphine gas.
As a rodenticide, aluminium phosphide pellets are provided as a mixture with food for consumption by the rodents. The acid in the digestive system of the rodent reacts with the phosphide to generate the toxic phosphine gas. Other pesticides similar to aluminium phosphide are zinc phosphide and calcium phosphide.
As a rodenticide, aluminium phosphide can be encountered under various brand names, e.g. Celphos, Fumitoxin, Phostoxin, and Quick Phos.
Incidents
In October 2002, Sir Derek Bibby, 2nd baronet and great-great-grandson of the founder and past chairman and president of the Bibby Line shipping company, aged 80 and terminally ill with leukaemia, committed suicide by consuming aluminium phosphide - the poison, hours later, caused his body to emit dangerous fumes forcing the evacuation of the hospital department where his body was being held.[5]
In February 2009, two children died in Jeddah, Saudi Arabia after a neighbouring house was fumigated with aluminium phosphide.[6]
Semiconductor applications
Industrially, AlP is a semiconductor material that is usually alloyed with other binary materials for applications in devices such as light-emitting diodes (e.g. aluminium gallium indium phosphide).[7]
References
- ↑ 1.0 1.1 1.2 Berger, L. I. Semiconductor materials; CRC Press, 1996; p 125. ISBN 0849389127.
- ↑ Index no. 015-004-00-8 of Annex VI, Part 3, to Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. OJEU L353, 31.12.2008, pp 1–1355 at p 372.
- ↑ White, Wayne E.; Bushey, A. H. Aluminum Phosphide. Inorg. Synth. 1953, 4, 23–25. DOI: 10.1002/9780470132357.ch7.
- ↑ Holleman, A. F.; Wiberg, E. Inorganic Chemistry; Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- ↑ Millionaire's death sparks poison scare; BBC News, 2002-10-10, <http://news.bbc.co.uk/2/hi/uk_news/england/2314911.stm>. (accessed 5 April 2009).
- ↑ Fumes kill two Danes in Jeddah; BBC News, 2009-02-24, <http://news.bbc.co.uk/2/hi/middle_east/7908102.stm>. (accessed 25 February 2009).
- ↑ Corbridge, D. E. C. Phosphorus: An Outline of its Chemistry, Biochemistry, and Technology, 5th ed.; Elsevier: Amsterdam, 1995. ISBN 0-444-89307-5.
External links
- International Chemical Safety Card 0472
- IPCS Environmental Health Criteria 194: Aluminium
- NLM Hazardous Substances Data Bank entry for Aluminum phosphide
| Error creating thumbnail: Unable to save thumbnail to destination | |
This page was originally imported from Wikipedia, specifically this version of the article "Aluminium phosphide". Please see the history page on Wikipedia for the original authors. This WikiChem article may have been modified since it was imported. It is licensed under the Creative Commons Attribution–Share Alike 3.0 Unported license. |