Difference between revisions of "Isobutanol"
Physchim62 (talk | contribs) (Imported from http://en.wikipedia.org/w/index.php?title=Isobutanol&oldid=301418086) |
Physchim62 (talk | contribs) (→External links) |
||
| Line 86: | Line 86: | ||
==External links== | ==External links== | ||
| − | * | + | {{Commons|Butanol}} |
| + | *{{EHC|65|name=Butanols: four isomers}} | ||
* http://www.forbes.com/2008/03/19/innovation-ethanol-fuel-tech-innovation08-cx_wp_0319innovation.html | * http://www.forbes.com/2008/03/19/innovation-ethanol-fuel-tech-innovation08-cx_wp_0319innovation.html | ||
| − | |||
| − | |||
[[Category:Alcohols]] | [[Category:Alcohols]] | ||
{{Imported from Wikipedia|name=Isobutanol|id=301418086}} | {{Imported from Wikipedia|name=Isobutanol|id=301418086}} | ||
Revision as of 08:35, 22 August 2009
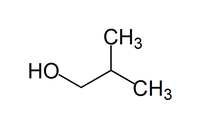
| Isobutanol | |
|---|---|
| |

| |
| IUPAC name | 2-Methylpropan-1-ol |
| Other names | Isobutyl alcohol, IBA, 2-methylpropyl alcohol |
| Identifiers | |
| CAS number | [] |
| RTECS | NP9625000 |
| SMILES | |
| Properties | |
| Chemical formula | C4H10O |
| Molar mass | 74.12 g/mol |
| Appearance | Colorless liquid |
| Density | 0.802 g/cm³, liquid |
| Melting point |
-108 °C (165 K) |
| Boiling point |
108 °C (381 K) |
| Solubility in water | Limited solubility |
| Viscosity | 3.95 centipoise at 20°C |
| Structure | |
| Hazards | |
| Material safety data sheet (MSDS) | External MSDS |
| R/S statements | R: 10-20-22-36-37-38 S: 7-16-24/25-26 |
| NFPA 704 | |
| Flash point | 28 °C |
| Related compounds | |
| Other alcohols | Isopropyl alcohol, ethanol, 2-butanol |
| Other compounds | acetone, propylene, diisopropyl ether |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Isobutanol (IUPAC nomenclature: 2-methylpropan-1-ol; also known as 2-methylpropyl alcohol, among other names) is a colorless, flammable, organic compound with a characteristic smell. Its isomers are n-butanol, 2-butanol, and tert-butanol. It is classified as an alcohol, and, as such, it is widely used as a solvent in chemical reactions, as well as being a useful starting material for organic synthesis.
Isobutanol is produced naturally during the fermentation of carbohydrates. It may also be a by-product of the decay process of organic matter.
Applications
- feedstock in the manufacture of isobutyl acetate, which is used in the production of lacquer and similar coatings, and in the food industry as a flavouring agent
- in the industrial synthesis of derivative esters; isobutyl esters such as diisobutyl phthalate (DIBP) are used as plasticizer agents in plastics, rubbers, and other dispersions
- as paint solvent
- as varnish remover
- as ink
- as paint additive, to reduce viscosity
- as paint additive, to improve brush flow
- as paint additive, to retard the formation of oil residues (blush) on painted surfaces
- as gasoline additive, to reduce carburetor icing
- as additive to automotive polish
- as additive to automotive paint cleaner
- as a chemical extractant in production of organic compounds
- as a mobile phase in thin layer chromatography.
Future applications could serve as an eventual substitute to gasoline to fuel combustion engines and is being aggressively researched by companies such as Gevo for this use.
Safety
Isobutanol is a volatile, flammable liquid that should be stored and used in well-ventilated areas. It is moderately irritating to the skin and greatly irritating to the eyes, mucous membranes, and respiratory tract. Exposure to high concentrations of its vapour can cause temporary narcosis.
Isobutanol is considered to be slightly toxic and it has been shown to cause liver damage in mice and humans. Ingestion may also lead to alcohol poisoning.
In March 2009 the Canadaian government announced its ban of isobutanol in cosmetics.[1]
MSDS
http://www.jtbaker.com/msds/englishhtml/i7600.htm
References
- ↑ Cosmetic Chemicals Banned in Canada Chemical & Engineering News, 87, 11 (16 March 2009), p. 38
- Health and Safety Guide for Isobutanol. World Health Organisation, 1987. Last accessed October 5, 2005.
External links
| Public domain and freely licensed images and media can be found in the corresponding category on Wikimedia Commons. |
- IPCS Environmental Health Criteria 65: Butanols: four isomers
- http://www.forbes.com/2008/03/19/innovation-ethanol-fuel-tech-innovation08-cx_wp_0319innovation.html
| Error creating thumbnail: Unable to save thumbnail to destination | |
This page was originally imported from Wikipedia, specifically this version of the article "Isobutanol". Please see the history page on Wikipedia for the original authors. This WikiChem article may have been modified since it was imported. It is licensed under the Creative Commons Attribution–Share Alike 3.0 Unported license. |
