tert-Butanol
| tert-Butanol | |
|---|---|

| |
| IUPAC name | 2-Methylpropan-2-ol |
| Other names | t-Butanol 2-Methyl-2-propanol t-Butyl alcohol tert-Butyl alcohol tertiary-Butyl alcohol 1,1-Dimethylethanol Dimethylethanol |
| Identifiers | |
| InChI | InChI=1/C4H10O/c1-4(2,3)5/h5H,1-3H3 |
| InChIKey | DKGAVHZHDRPRBM-UHFFFAOYAF |
| Standard InChI | InChI=1S/C4H10O/c1-4(2,3)5/h5H,1-3H3 |
| Standard InChIKey | DKGAVHZHDRPRBM-UHFFFAOYSA-N |
| CAS number | [] |
| EC number | |
| RTECS | EO1925000 |
| ChemSpider | |
| SMILES | |
| Properties[1][2][3][4][5] | |
| Chemical formula | C4H9OH |
| Molar mass | 74.122 g/mol |
| Appearance | colorless liquid, semi-solid or solid |
| Density | 0.7809 g/cm3 |
| Melting point |
25 °C, 298.3 K, 77 °F |
| Boiling point |
82 °C, 355.5 K, 180 °F |
| Triple point | 298.96 K |
| Critical point | 506.2 K, 39.7 bar |
| Thermochemistry[2][3][6] | |
| Std enthalpy of formation ΔfH |
−359 kJ/mol, liquid |
| Std enthalpy of combustion ΔcH |
−2644 kJ/mol, liquid −2633 kJ/mol, solid |
| Standard molar entropy S |
193.5 J K−1 mol−1, liquid 170.9 J K−1 mol−1, solid |
| Specific heat capacity C | 146.1 J K−1 mol−1, solid |
| Std enthalpy of vaporization ΔvapH |
46.5 kJ/mol |
| Hazards[5][7][8] | |
| Material safety data sheet (MSDS) | ICSC |
| EU index number | 603-005-00-1 |
| GHS pictograms |  
|
| GHS signal word | DANGER |
| GHS hazard statements | H225, H332 |
| Flash point | 11 ºC (52 ºF) |
| Autoignition temp. | 470 ºC (878 ºF) |
| Explosive limits | 2.4–8.0% |
| PEL (U.S.) | 100 ppm TWA |
| IDLH level | 1600 ppm |
| Related compounds | |
| Other butanols | Butan-1-ol Isobutanol sec-Butanol |
| Other compounds | 2-Methylbutan-2-ol |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
tert-Butanol, or 2-methyl-2-propanol (colourless liquid or white solid, depending on the ambient temperature), is the simplest tertiary alcohol. It is one of the four isomers of butanol. tert-Butanol is a clear liquid with a camphor-like odor. It is very soluble in water and miscible with ethanol and diethyl ether. It is unique among the isomers of butanol because it tends to be a solid at room temperature, with a melting point slightly above 25 °C.
Contents
Preparation
tert-Butanol is derived commercially from isobutane as a co-product of propylene oxide producion. It can also be produced by the catalytic hydration of isobutylene.
Applications
tert-Butanol is used as a solvent, as a denaturant for ethanol, as an ingredient in paint removers, as an octane booster for gasoline, as an oxygenate gasoline additive, and as an intermediate in the synthesis of other chemical commodities such as MTBE, ETBE, TBHP, other flavors and perfumes.
Chemistry
As a tertiary alcohol, tert-butanol is more stable to oxidation and less reactive than the other isomers of butanol.
When tert-butanol is deprotonated with a strong base, the product is an alkoxide anion. In this case, it is tert-butoxide. For example, the commonly used organic reagent potassium tert-butoxide is prepared by refluxing dry tert-butanol with potassium metal.[9]
- K + t-BuOH → t-BuO−K+ + ½H2
The tert-butoxide species is itself useful as a strong, non-nucleophilic base in organic chemistry. It is able to abstract acidic protons from the substrate molecule readily, but its steric bulk inhibits the group from participating in nucleophilic substitution, such as in a Williamson ether synthesis or an SN2 reaction.
Conversion to alkyl halide
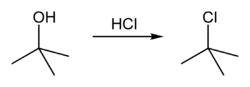
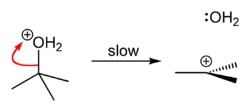
tert-Butanol (also tert-butyl alcohol) reacts with hydrogen chloride to form tert-butyl chloride and water via an SN1 mechanism.
 |
 |
 |
The overall reaction, therefore, is:
Because tert-butanol is a tertiary alcohol, the relative stability of the tert-butyl carbocation in the Step 2 allows the SN1 mechanism to be followed. Primary alcohols generally undergo an SN2 mechanism because the relative stability of a primary carbocation intermediate is very low. The tertiary carbocation in this case is able to stabilize itself better due to electron donation from surrounding sp3 hybridized carbons.
References
- ↑ The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 11th ed.; Merck, 1989. ISBN 091191028X, 1542.
- ↑ 2.0 2.1 Ethanol, 1,1-dimethyl-. In NIST Chemistry WebBook; National Institute for Standards and Technology, <http://webbook.nist.gov/cgi/inchi/InChI%3D1S/C4H10O/c1-4(2,3)5/h5H,1-3H3>.
- ↑ 3.0 3.1 Oetting, F. L. The heat capacity and entropy of 2-methyl-2-propanol from 15 to 330ºK. J. Phys. Chem. 1963, 67 (12), 2757–61. DOI: 10.1021/j100806a059.
- ↑ Ambrose, D.; Townsend, R. Thermodynamic Properties of Organic Oxygen Compounds IX. The Critical Properties and Vapor Pressures Above Five Atmospheres of Six Aliphatic Alcohols. J. Chem. Soc. 1963, 3614–25. DOI: 10.1039/JR9630003614. Majer, Vladimír; Svoboda, Václav Enthalpies of Vaporization of Organic Compounds: A Critical Review and Data Compilation; Blackwell: Oxford, 1985. ISBN 0632015292. Wilhoit, Randolph C.; Chao, Jing; Hall, Kenneth R. Thermodynamic Properties of Key Organic Compounds in the Carbon Range C1 to C4. Part 1. Properties of Condensed Phases. J. Phys. Chem. Ref. Data 1985, 14, 1–175. DOI: 10.1063/1.555747. Gude, Michael; Teja, Amyn S. Vapor-Liquid Critical Properties of Elements and Compounds. 4. Aliphatic Alkanols. J. Chem. Eng. Data 1995, 40 (5), 1025–36. DOI: 10.1021/je00021a001.
- ↑ 5.0 5.1 tert-Butanol; International Chemical Safety Card 0114; International Labour Organization: Geneva, March 1995, <http://www.inchem.org/documents/icsc/icsc/eics0114.htm>.
- ↑ Parks, George S.; Anderson, C. Travis Thermal data on organic compounds. III. The heat capacities, entropies and free energies of tertiary butyl alcohol, mannitol, erythritol and normal butyric acid. J. Am. Chem. Soc. 1926, 48 (6), 1506–12. DOI: 10.1021/ja01417a009. Parks, George S.; Kelley, Kenneth K.; Huffman, Hugh M. Thermal data on organic compounds. V. A revision of the entropies and free energies of nineteen organic compounds. J. Am. Chem. Soc. 1929, 51 (7), 1969–73. DOI: 10.1021/ja01382a003. Raley, John H.; Rust, Frederick F.; Vaughan, William E. Decompositions of Di-t-alkyl peroxides. I. Kinetics. J. Am. Chem. Soc. 1948, 70 (1), 88-94. DOI: 10.1021/ja01181a027. Skinner, H. A.; Snelson, A. The heats of combustion of the four isomeric butyl alcohols. Trans. Faraday Soc. 1960, 56, 1776–83. DOI: 10.1039/TF9605601776. Wiberg, Kenneth B.; Hao, Shide Enthalpies of hydration of alkenes. 4. Formation of acyclic tert-alcohols. J. Org. Chem. 1991, 56 (17), 5108–10. DOI: 10.1021/jo00017a022.
- ↑ Index no. 603-005-00-1 of Annex VI, Part 3, to Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. OJEU L353, 31.12.2008, pp 1–1355 at p 477.
- ↑ tert-Butyl alcohol. In Pocket Guide to Chemical Hazards; U.S. Department of Health and Human Services (NIOSH) Publication No. 2005-149; Government Printing Office: Washington, DC, 2005. ISBN 9780160727511, <http://www.cdc.gov/niosh/npg/npgd0078.html>.
- ↑ Johnson, William S.; Schneider, William P. β-Carbethoxy-γ,γ-diphenylvinylacetic acid. Org. Synth. 1950, 30, 18, <http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv4p0132>; Coll. Vol., 4, 132
External links
- International Chemical Safety Card 0114
- NIOSH Pocket Guide to Chemical Hazards 0078
- IPCS Environmental Health Criteria 65: Butanols: four isomers
- IPCS Health and Safety Guide 7: tert-Butanol
| Error creating thumbnail: Unable to save thumbnail to destination | |
This page was originally imported from Wikipedia, specifically this version of the article "Tert-Butanol". Please see the history page on Wikipedia for the original authors. This WikiChem article may have been modified since it was imported. It is licensed under the Creative Commons Attribution–Share Alike 3.0 Unported license. |